New methods for reducing air pollution and generating solar fuels developed by scientists at City University of Hong Kong (CityU) offer practical solutions to the energy shortage, environmental issues, and related public health risks.


The research has been generated by two projects led by Dr Ng Yun-hau, Associate Professor, and Dr Shang Jin, Assistant Professor, respectively, in the School of Energy and Environment (SEE). The research has been published in the top chemistry journal Angewandte Chemie.
Dr Ng and his team have designed a new solar-powered catalyst that can convert carbon dioxide (CO2) into methane fuel through artificial photosynthesis. Their work is published in a paper titled “Metal-Organic Frameworks Decorated Cuprous Oxide Nanowires for Long-lived Charges Applied in Selective Photocatalytic CO2 Reduction to CH4”.
“Methane is a major component of domestic fuel gases. Turning CO2 into methane fuel using sunlight has the potential to produce a clean and sustainable energy alternative, thereby reducing our carbon emissions and reliance on fossil fuels,” Dr Ng said.
However, the key problems with CO2 conversion are short excited charges in the lifetime of the catalyst and non-selective reduction. Cuprous oxide (Cu2O), commonly used for CO2 conversion, undergoes self-corrosion after brief illumination, and it creates an array of product mixture from the reduction process, hindering large scale application.
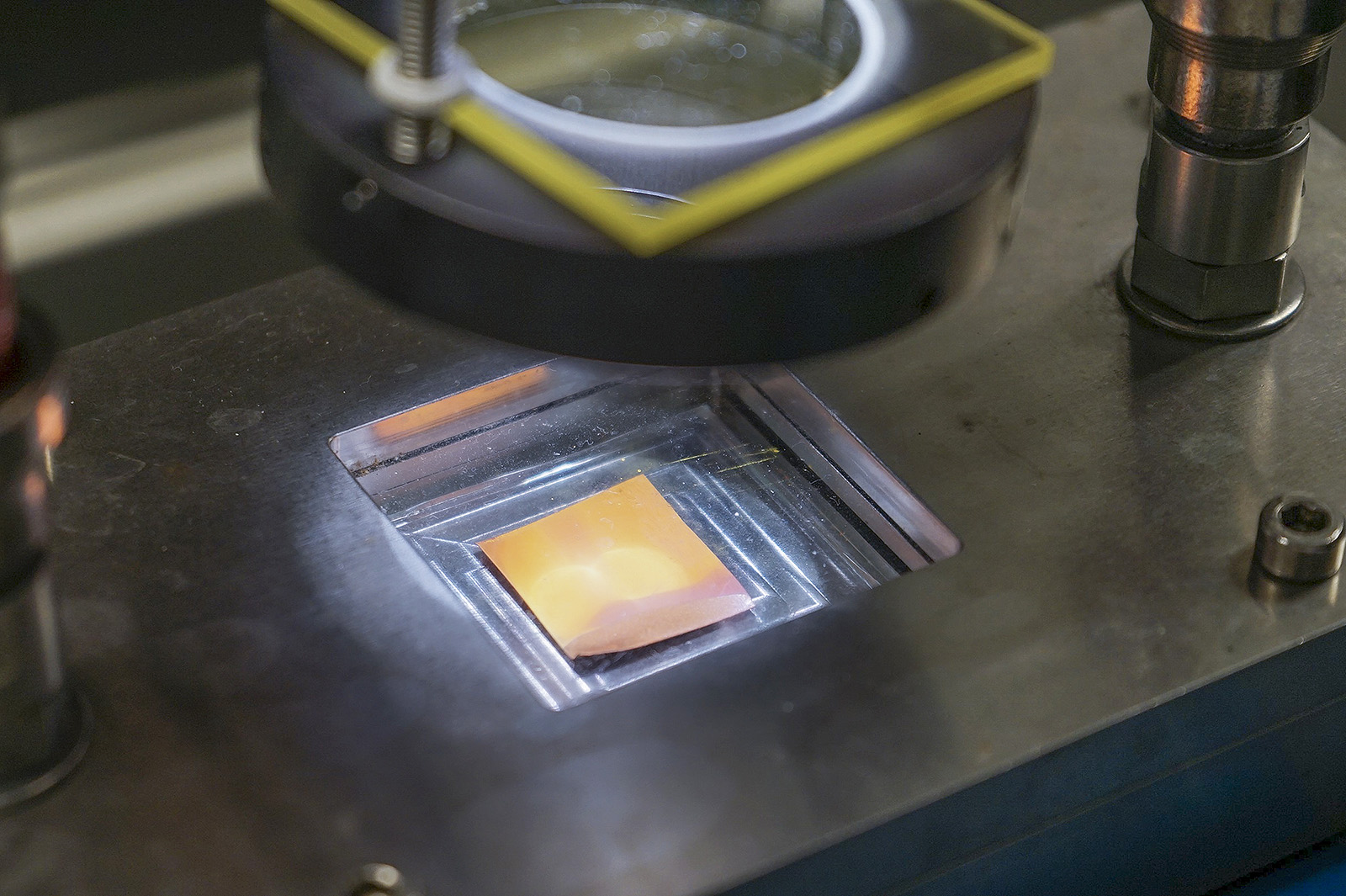

Dr Ng’s team has solved these problems by uniformly enwrapping Cu2O with a copper-based metal-organic framework (MOF) at the microscopic level. This MOF, which is a good CO2 adsorbent, strengthens the interaction between the CO2 and the catalyst, enabling a higher concentration of CO2 on the surface of catalyst. The team unveiled for the first time the presence of charge transfer between MOF and cuprous oxide, which can prolong the charges lifetime by ten times for higher activity. With the conformal coating of MOF, the Cu2O becomes stable and its corrosion is delayed.
“We hope we can recycle the unwanted CO2 from industry and transportation sectors at an affordable cost in the future and use it as the precursor to produce green and alternative fuels. We will continue to explore ways to further increase the methane production rate and scale up the catalyst synthesis and the reactor systems,” said Dr Ng.
Dr Ng is the corresponding author of the paper. The first is Dr Wu Hao, Postdoctoral Fellow from SEE. Other collaborating researchers are from University College London, University of New South Wales, Monash University Malaysia, and Swinburne University of Technology.
The other study, carried out by the team led by Dr Shang, aims to control pollution resulting from nitrogen dioxide (NO2), a major roadside pollutant causing photochemical smog and damage to the human respiratory tract. The team revealed a new class of robust adsorbent materials for capturing ambient NO2 in a paper titled “Transition‐Metal‐Containing Porphyrin Metal–Organic Frameworks as π‐Backbonding Adsorbents for NO2 Removal”.
The team has developed a series of sponge-like nano porous materials featuring tailored transition metals as active sites at the porphyrin rings, which can selectively bind and remove NO2 from gas mixtures. The concept was inspired by the pi-backbonding interaction in the human body, through which the iron metal at the porphyrin of the hemoglobin protein can selectively bind oxygen molecules where pi-backbonding occurs.


This novel adsorption-based technology complements the conventional selective catalytic reduction method, which is applicable only to NO2 conversion at high temperatures (about 250 to 600 °C). It can mitigate ambient NO2 pollution from low-temperature exhaust, such as that generated by off-road vehicles.
“Our successful demonstration of selective NO2 adsorption in ambient temperature is conducive to the development of a series of technologies for low-temperature NO2 pollution control, such as sensing, filtration, and catalysis of low-temperature NO2, in particular from environmental hotspots, including tunnels and semi-confined car parks,” said Dr Shang.
The research results showed that the adsorbent has high stability, selectivity, capacity, and regenerability. It is resistant to corrosion and is not affected by humidity. Also, it can be made into different forms based on its application, such as spherical shapes for use in ventilation systems or filters for respirator masks.
Dr Shang and Dr Gu Qinfen from the Australian Synchrotron research facility are the corresponding authors of the paper. The first is Shang Shanshan, a PhD student from SEE. The study involved collaboration among researchers at the University of Hong Kong, the Guangzhou Institute of Energy Conversion at the Chinese Academy of Sciences, and Jilin University.








































