A research team led by Assistant Professor Dai Yunlu in the University of Macau (UM) Faculty of Health Sciences (FHS) has developed a novel metal-phenolic network to potentiate the activation of stimulator of interferon genes (STING) pathway within tumour microenvironment for advanced radiotherapy. The novel nanomedicine is expected to improve the efficacy of immunotherapy. The research findings have been published in Advanced Materials.
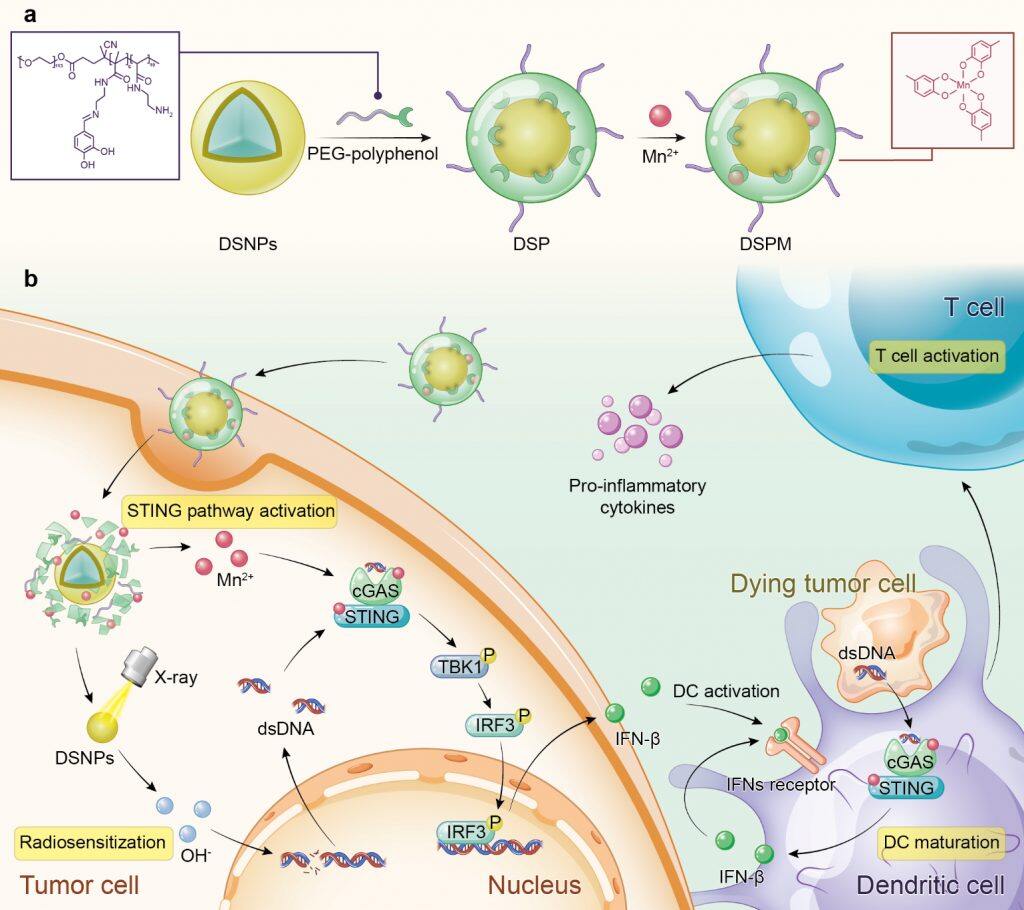

In recent years, new developments in immunotherapy have revolutionised cancer treatment. However, the ‘cold’ tumour microenvironment caused by low tumour-infiltrating lymphocytes results in a low response rate of cancer immunotherapy. Recent studies have shown that the activation of STING pathway can activate innate immunity and turn a ‘cold’ T cell-infiltrated tumour microenvironment into a ‘hot’ one, which can help to improve the efficacy of immunotherapy for oncology patients. Traditional radiotherapy generates cytosolic dsDNA to evoke STING pathway activation. Additionally, MnCl2 alone can act as an activator of the STING pathway. The research team engineered a novel metal-phenolic network via the coordination interactions between phenolic ligands and metal ions, potentiating STING pathway activation within tumour microenvironment for advanced radiotherapy.


In this study, the amphiphilic polymer PEG-polyphenol was introduced to coordinate with lanthanide-doped radiosensitizer (DSNPs) and Mn2+ to form stabilised metal-phenolic networks DSPM. After cell internalisation, DSPM disassembled owing to the cleavage of imine linkers in PEG-polyphenol, imparting the release of DSNPs and Mn2+. Upon X-ray irradiation, DSNPs sensitised cancer cells and promoted cytosolic dsDNA release. The released Mn2+ facilitated the recognition of cytosolic dsDNA by cGAS, triggering the activation of the STING pathway and DC maturation. Subsequently, tumour-specific antigen was presented to T cells to evoke robust antitumor immunity. The 4T1 tumour-bearing mice model showed that DSPM combined with X-ray irradiation elevated the recruitment of tumour-infiltrating lymphocytes and the secretion of proinflammatory cytokines, promoting the successful transformation of the nonimmunogenic ‘cold’ microenvironment into an immunogenic ‘hot’ tumour microenvironment.
Prof Dai is the corresponding author of the study. Doctoral students Yan Jie and Wang Guohao, as well as FHS post-doctoral fellow Xie Lisi, are the co-first authors. Doctoral students Tian Hao, Sang Wei, Li Wenxi, and Zhang Zhan, as well as post-doctoral fellows Li Jie and Li Bei, also made important contributions to this study. This study was funded by the National Natural Science Foundation of China (file number: 32171318 and 32101069), the Start-up Research Grant (SRG) of UM (file number: SRG2018-00130-FHS), Ministry of Education Frontiers Science Center for Precision Oncology, the Science and Technology Development Fund, Macao SAR (file number: 0109/2018/A3、0011/2019/AKP、0113/2019/A2 and 0103/2021/A), and Shenzhen Science and Technology Innovation Commission, Shenzhen-Hong Kong-Macau Science and Technology Plan C (file number: SGDX20201103093600004). The full version of the research article can be viewed at: https://doi.org/10.1002/adma.202105783
Source: Faculty of Health Sciences, University of Macau (UM)