The University of Queensland is leading a trial across Brisbane and the Sunshine Coast focusing on improving the delivery of life-saving treatments for hospitalised babies.
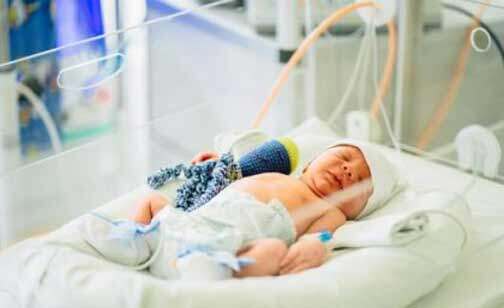

Professor Amanda Ullman from UQ’s School of Nursing, Midwifery and Social Work and Children’s Health Queensland said a new monitoring device will be tested to determine if it accurately detects when IV fluid is delivered into the tissue, instead of the vein.
“More than 18,000 babies younger than 12 months old are admitted to hospitals in Australia every year, with 60 per cent requiring an IV or ‘drip’ for treatments like rehydration and antibiotics,” Professor Ullman said.
“Our global studies have found 33 to 45 per cent of these IV’s stop working before the treatment is completed.
“The vein where the IV is placed can be damaged during treatment, resulting in the fluid pooling in the tissue rather than being administered into the bloodstream.
“Our trial will use an IV Biosensor – which includes a patient monitor, reusable electronic cable and a near infra-red sensor attached to the patient’s skin near the IV site – to continuously monitor the surrounding tissue.
“The IV Biosensor provides audible and visual alarms when tissue fluid volume changes.”
The research team will recruit more than 500 babies over 3 years to test the device at the Queensland Children’s Hospital, Royal Brisbane and Women’s Hospital Neonatal Unit, and Sunshine Coast University Hospital Neonatal Unit and Child and Adolescent Unit.
Professor Ullman said families who participate in the trial will be randomly assigned to receive either standard care or standard care plus the IV Biosensor.
“Babies will be carefully monitored to determine whether the IV Biosensor helps to detect when an IV isn’t working as it should be,” Professor Ullman said.
“Even with the best care and monitoring, unintentional injuries from IV’s can occur because by the time signs of injury appear such as pain, redness and swelling, the damage has already happened.
“Our aim is to detect IV issues as early as possible to prevent any potential consequences for babies.
“If effective, the new monitoring device could improve the experiences of babies and families throughout their hospital stay.”
The trial is funded by the 2022 Medical Research Future Fund Clinician Researchers – Nurses, Midwives and Allied Health Grant.