A number of years of careful laboratory tests have now led to the first pre-clinical tests of implantable biodegradable heart valves and stents.

Non-degradable prostheses for cardiovascular tissues can be used to replace heart valves and blood vessels, but they can’t stay in the body permanently. In two recent papers, TU/e researchers in collaboration with a number of clinical partners, the Dutch Heart Foundation, and TU/e spin-off companies Suprapolix, Xeltis, and STENTiT have shown how replacement stents and heart valves made from biodegradable materials can be a permanent solution as they help the body to replace damaged tissues. It’s the first time that these biodegradable heart valves and stents from their research have been evaluated in a pre-clinical setting.
Thanks to significant advancements in methods and materials, cardiovascular tissues such as heart valves and blood vessels compromised by disease or congenital malformations in the case of children can now be replaced with prostheses.
However, the majority of current prostheses are passive in that they do not help cells to grow near the implant and the non-degradable prostheses stay in the body permanently. As a result, these prostheses can affect patient quality of life over time. When implanted in children with congenital malformations, the prosthesis cannot grow with the body, which makes further operations inevitable. Therefore, there is considerable need for more permanent prosthetic replacements.
With this in mind, a group of TU/e researchers, led by Carlijn Bouten and Anthal Smits from the department of Biomedical Engineering, have explored the implantation of biodegradable plastic heart valves and blood vessels that are accepted by the body and transformed into living tissue in pre-clinical trials. They recently published their results in two significant paperin the journal JACC: Basic to Translational Science.
These studies are significant given that it has taken a number of years of careful laboratory developments by the researchers to reach this crucial stage where, for the first time, biodegradable heart valves and stents from their research have been evaluated in a pre-clinical setting.
CARDIOVASCULAR TISSUES FOR YOUNG AND OLD ALIKE
“These papers showcase some of the first translations of our basic engineering research in the field of regenerative medicine for implantable heart valves and blood vessels,” says Bouten. “For heart valves, we are targeting young children who are still growing since the current non-degradable heart valves do not work for them. Then we are designing regenerative stents for adults and elderly subjects with vascular diseases. This new stent is different from the current stents as it triggers the body to heal the damaged artery from the inside, while the stent structure itself only stays temporarily in the body. This can overcome many of the risks associated with permanent stents.”
“In both studies, the work took more than three years, but we are finally harvesting the tremendous efforts of our large interdisciplinary collaborations,” adds Smits. “Exceptionally, and accidentally, the papers were published within a week of each other.”
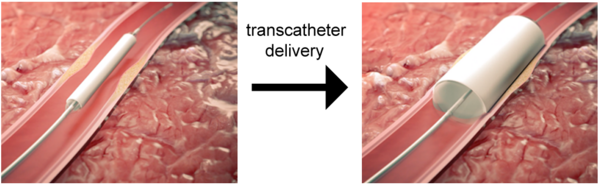

THE PROMISE OF REGENERATIVE STENTS
The gold standard for the replacement of diseased arteries or veins is the patient’s own arteries or veins. However, the use of such autologous vessels (vessels from the same person) is not always possible due to vascular disease, previous use of potential vessels, or when multiple or lengthy bypasses are necessary.
Although synthetic vessels made from nondegradable polymers are available, they work best for the replacement of large arteries (diameters greater than 6 mm). Smaller diameter implants, such as the coronary arteries or arteries in the lower leg, have a risk of getting blocked. This is also an important risk to consider when using nondegradable vascular stents to open up blocked arteries.
To address this issue, researchers from TU/e and the spin-off company STENTiT developed a small-diameter vascular implant made from a biodegradable electrospun polymer that can be inserted into a vessel using the same minimally invasive approach as transcatheter replacement.
The implant fulfilled a number of key roles. First, the biodegradable electrospun fibrous microstructure of the implant provided a suitable environment for the colonization and growth of the patient’s own cells. Second, the combination of the bioresorbable polymer and the graft design helped the stent to exert the necessary force to secure its position against the vascular wall tissue. Finally, the implant had a positive impact on tissue remodeling.
“The results from this pre-clinical study are hugely promising for future patient applications,” says Bouten. “It offers the possibility of implanting biodegradable stents in patients in a minimally invasive way that provide better structural support in vessels, and replicate the conditions needed by cells to flourish in situ.”
You can read the full paper in relation to the biodegradable heart stents here.
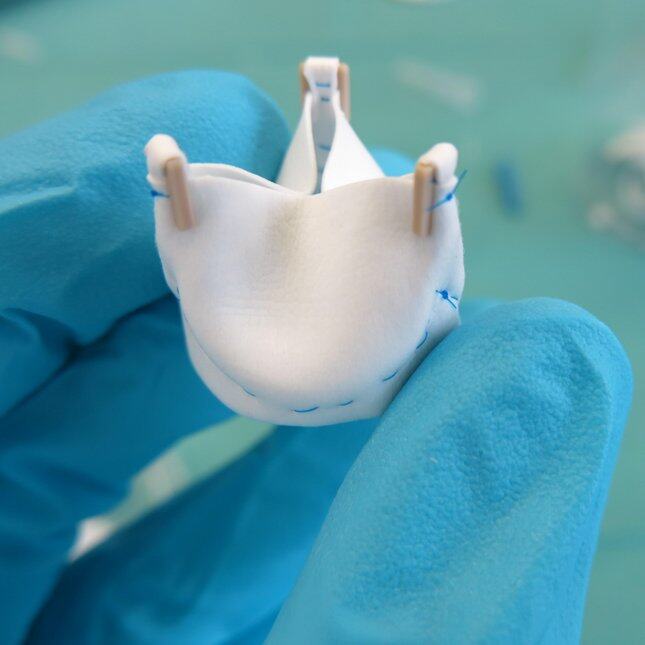

RESORBABLE HEART VALVES
In the second paper, an extensive collaboration co-led by TU/e and Amsterdam University Medical Center looked at the development of soluble heart valves.
Heart valve function strongly depends on the organization of the underlying collagen network. In mature heart valves, this network typically forms circles in the tissue. This preferred network orientation ensures the mechanical strength of the valve.
Non-living or synthetic valves can be used to replace diseased heart valves, but they are unable to promote tissue formation and fully integrate with the surrounding tissue. This negatively affects the quality of life and life expectancy of the patient.
An alternative is the use of cell-free biodegradable heart valves, which promote colonization of host cells in the valve structure, tissue formation, and remodeling. Simultaneously, the biodegradable scaffold is then slowly absorbed by the body.
The researchers produced electrospun valvular scaffolds from resorbable elastomers, with the aim of studying how different scaffolds with different network alignments affected new tissue growth. One of the main findings is that the implanted valves were capable of sustaining valve functionality for up to 1 year, while the plastic implant was being degraded and replaced by new tissue that was regenerated by the body itself. Surprisingly, however, none of the scaffolds led to a collagen network consisting of circles in the tissue as would be seen in normal heart valve tissue.
“The observation that the valves can continuously open and close properly throughout the process of being regenerated is very important,” says Smits. “However, this pre-clinical study also underlines that we still need a better understanding of the body’s response to our implants to gain full control over the tissue that is regenerated. The next step will involve careful revision of scaffold designs using data from further pre-clinical trials.”
You can read the full paper in relation to resorbable heart valves here.







































